Here is the correct question.
You calculate the Wilson equation parameters for the ethanol (1) +1 - propanol (2) system at 25° C and find they are ∧₁₂ = 0.7 and ∧₂₁ = 1.1 . Estimate the value of parameters at 50° C
Answer:
the values of the parameters at 50° C are 0.766 and 1.047
Step-by-step explanation:
From "critical point , enthalpy of phase change and liquid molar volume " the liquid molar volume (v) of ethanol and 1 - propanol is represented as follows:
Compound Liquid molar volume (cm³/mol)
Ethanol (1) 58.68
1 - propanol (2) 75.14
To calculate the temperature dependent parameters of the Wilson equation ∧₁₂.
∧₁₂ =
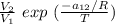 ------------ equation (1)
------------ equation (1)
where:
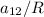 = Wilson parameter = ???
= Wilson parameter = ???
 = liquid molar volume of component 2 = 75.14 cm³/mol
= liquid molar volume of component 2 = 75.14 cm³/mol
 = liquid molar volume of component 1 = 58.68 cm³/mol
= liquid molar volume of component 1 = 58.68 cm³/mol
T = temperature = 25° C = ( 25 + 273.15) K = 298.15 K
Replacing our values in the above equation ; we have:
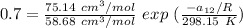
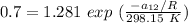
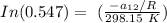
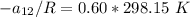
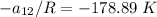
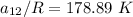
To calculate the temperature dependent parameters of the Wilson equation ∧₂₁
∧₂₁ =
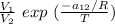 ---------- equation (2)
---------- equation (2)
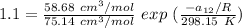
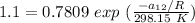
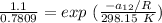
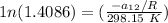
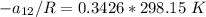
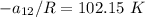
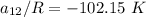
From equation (1) ; let replace 178.98 K for
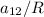
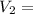 75.14 cm³/mol
75.14 cm³/mol
 = 58.68 cm³/mol
= 58.68 cm³/mol
T = 50° C = ( 50 + 273.15 ) K = 348.15 K
So;
∧₁₂ =
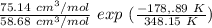
∧₁₂ = 1.281 exp(-0.5138)
∧₁₂ = 1.281 × 0.5982
∧₁₂ =0.766
From equation 2; let replace 102.15 K for
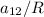
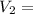 75.14 cm³/mol
75.14 cm³/mol
 = 58.68 cm³/mol
= 58.68 cm³/mol
T = 50° C = ( 50 + 273.15 ) K = 348.15 K
So;
∧₂₁ =
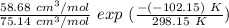
∧₂₁ = 0.7809 exp (0.2934)
∧₂₁ = 0.7809 × 1.3410
∧₂₁ = 1.047
Thus, the values of the parameters at 50° C are 0.766 and 1.047