Answer:
Molarity of unknown
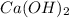 solution is 0.304 M.
solution is 0.304 M.
Step-by-step explanation:
Neutralization reaction:
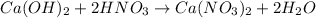
So, 2 moles of
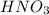 neutralize 1 mol of
neutralize 1 mol of
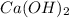
Moles of
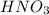 added =
added =
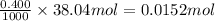
So, 0.0152 mol of
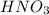 neutralize 0.00760 mol of
neutralize 0.00760 mol of
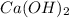
Let's assume molarity of
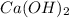 is C (M)
is C (M)
Then number of moles of
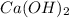 in 25.00 mL of C (M) of
in 25.00 mL of C (M) of
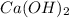
=
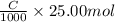
Hence,
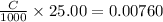
or, C = 0.304
So, molarity of unknown
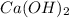 solution is 0.304 M
solution is 0.304 M