Answer: 12.0 milliliters of 6.50 M HCl ( aq ) are required to react with 2.55 g Zn.
Step-by-step explanation:
moles =
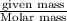
moles of zinc =
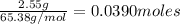
The balanced chemical equation is :
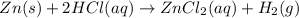
According to stoichiometry:
1 mole of zinc reacts with = 2 moles of HCl
Thus 0.0390 moles of zinc reacts with =
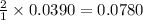 moles of HCl
moles of HCl
To calculate the volume for given molarity, we use the equation:
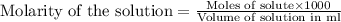 .....(1)
.....(1)
Molarity of
 solution = 6.50 M
solution = 6.50 M
Volume of solution = ?
Putting values in equation 1, we get:
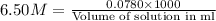
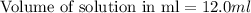
Thus 12.0 ml of 6.50 M HCl ( aq ) are required to react with 2.55 g Zn