5 grams of water will have more water molecules since ice had 1.67 x
 molecules and 5 gm ice had 1.53 x 10
molecules and 5 gm ice had 1.53 x 10
 molecules of water.
molecules of water.
Step-by-step explanation:
5 liters of water at 0 degrees or 273.15 K will have, Formula used is:
number of water molecules =
 x Avagadro number
x Avagadro number
Avagadro number = 6.022 X 10 ^23 molecules or atoms in 1 mole
atomic mass of 1 mole water = 18.01 grams/mole
putting the value in the equation:
number of molecules =
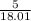 x 6.022 x 10^23
x 6.022 x 10^23
number of molecule of water in water = 1.67 X
 molecules.
molecules.
5 gm cm^3 of ice will have
The density of ice = 0.917 gm/cm^3
volume = density x mass
number of moles of water =
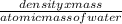 x Avagadro number
x Avagadro number
putting the values,
number of moles in ice =
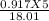 X 6.022 X 10^23
X 6.022 X 10^23
= 1.53 X
 molecules of water
molecules of water