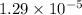Answer:
The value of specific heat is
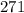
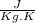 and coefficient of thermal expansion is
and coefficient of thermal expansion is
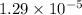
Step-by-step explanation:
Given:
Density of alloy
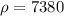
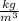
For finding the specific heat of alloy we use formula of specific heat in case of solid material,
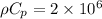
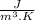
Where
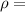 density of alloy,
density of alloy,
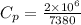
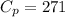
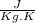
For finding the coefficient of thermal expansion,
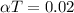
Where
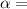 coefficient of thermal expansion
coefficient of thermal expansion
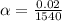
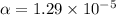
Therefore, the value of specific heat is
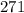
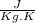 and coefficient of thermal expansion is
and coefficient of thermal expansion is