Answer:
126. g/mol is the molar mass of the acid .
Step-by-step explanation:
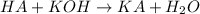
Moles of KOH = n
Volume of the solution of KOH = 19.0 mL = 0.019 L
Molarity of the KOH solution = 0.260 M
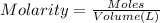
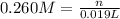
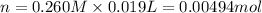
Accrding to recation, 1 mol KOH react with 1 mole of HA ,then 0.00494 mol of KOH will react with :
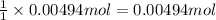 of HA
of HA
Mass of HA = 0.623 g
Molar mass of HA = M
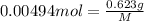
[tex[M=\frac{0.623 g}{0.00494 mol}=126. g/mol[/tex]
126. g/mol is the molar mass of the acid .