Answer:
(A)
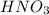 Solubility will increase.
Solubility will increase.
(B)
 Solubility will decrease.
Solubility will decrease.
(C)
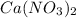 Solubility will decrease.
Solubility will decrease.
Step-by-step explanation:
a) According to Le Chatelier's Principle when a change is introduced in a reaction system at equilibrium, the system responds by the reaction shifting in the direction to counter the change introduced.
When the change introduced is addition of acid, some
 are consumed due to it and the system would respond by reaction shifting towards creation of more
are consumed due to it and the system would respond by reaction shifting towards creation of more
 in the system by increased dissolution. Thus solubility increase.
in the system by increased dissolution. Thus solubility increase.
(b) When
 are added, the reaction shifts to counter the change introduced of increased
are added, the reaction shifts to counter the change introduced of increased
 by shifting to side that decreases the increased
by shifting to side that decreases the increased
 . That happens by reaction shifting towards some
. That happens by reaction shifting towards some
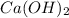 precipitating back. Hence solubility decreases.
precipitating back. Hence solubility decreases.
(c) The change introduced is addition of
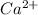 which will be countered by reaction shifting to side that decreases increased
which will be countered by reaction shifting to side that decreases increased
 , which happens by some
, which happens by some
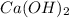 precipitating back. Hence solubility decreases.
precipitating back. Hence solubility decreases.