Step-by-step explanation:
Given that,
Diameter of the oil droplet,
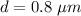
Radius,
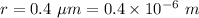
Separation between the electrodes, d = 11 mm
The droplet hangs motionless if the upper electrode is 22.8 V more positive than the lower electrode.
The density of the oil is
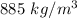
(a) Density of oil is given by mas per unit volume.
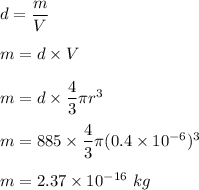
(b) The electric force is balanced by the mass of the object. So,
qE = mg
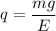
E is electric field, E = V/d
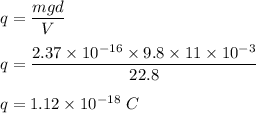
(c) Charges come in integer multiples of the electronic charge units of e. So,
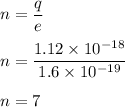
Hence, this is the required solution.