0.73 M is the concentration of sulfuric acid that needed 47 mL of 0.39M potassium hydroxide solution to neutralize a 25 mL sample of the sulfuric acid solution.
Step-by-step explanation:
Data given:
Volume of the base = 47 ml
molarity of the base= 0.39 M
volume of the acid = 25 ml
molarity of the acid =?
For titration reaction between acid and base, the volume or molarity of any of the base or acid can be determined. The formula used:
Macid X Vacid = Vbase x Mbase
Macid =
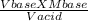
putting the values given in the rearranged equation above:
Macid =
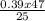
= 0.73 M
The concentration of the sulphuric acid needed is 0.73 M.