Answer:
Step-by-step explanation:
1. Balanced chemical equation
- Ba(OH)₂(aq) + 2HNO₃(aq) → Ba(NO₃)₂(aq) + 2H₂O(l)
2. Determine the number of moles of HNO₃ in the solution
- n = M × V = 0.425M × 0.050 liter = 0.02125 mol HNO₃
3. Use the mole ratio from the balanced chemical equation and the number of moles of HNO₃ to determine the number of moles of Ba(OH)₂.
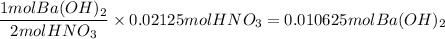
4. Determine the molar concentration of the solution of Ba(OH)₂
- M = 0.010625mol/0.0368liter