This is an incomplete question, here is a complete question.
An object has a mass of 0.125 lb. When it is submerged in a graduated cylinder initially containing 19.6 mL of water, the water level rises to 39.9 mL. What is the density (g/mL) of the object?
(given 2.2 lb = 1.0 kg, 1000 g =1.0 kg)
Answer : The density of object is, 2.79 g/mL
Explanation :
First we have to calculate the mass of object in terms of grams.
Conversion used:
2.2 lb = 1 kg = 1000 g
That means,
2.2 lb = 1000 g
So, 0.125 lb =
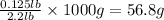
Now we have to calculate the volume of object.
Volume of object = Volume of water level rises - Initial volume of water
Volume of object = 39.9 mL - 19.6 mL
Volume of object = 20.3 mL
Now we have to calculate the density of object.
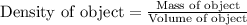
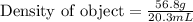
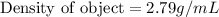
Therefore, the density of object is, 2.79 g/mL