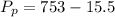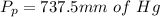Complete Question
The complete question is shown on the first uploaded image
Answer:
The partial pressure is
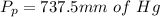
Step-by-step explanation:
The Partial pressure of
 is mathematically represented as
is mathematically represented as
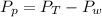
Where
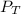 is the total pressure of water with a value of 15.5 mm of Hg
is the total pressure of water with a value of 15.5 mm of Hg
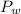 is the partial pressure of water with a value 753 mm of Hg
is the partial pressure of water with a value 753 mm of Hg
Now substituting values