Answer:
The number of formula units of silver nitrate is
 .
.
Step-by-step explanation:
Moles of silver nitrate = n = 7.65 mol
1 mole =
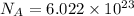 atoms/ molecules/ ions
atoms/ molecules/ ions
Let the number of formula units of silver nitrate be N.
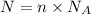
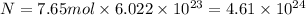
The number of formula units of silver nitrate is
 .
.