Answer:
0.8929 mol
Step-by-step explanation:
An 11.2-L sample of gas is determined to contain .50 mol N2. at the same temperature and pressure, how many moles of gas would there be in a 20. -L sample?
According to avogadro's law
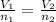
From the given data
V1 = 11.2L
n1 = 0.5mole
V2 = 20L
n2 =?
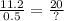
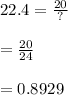
= 0.8929 mol