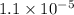Answer : The value of acid dissociation constant is,
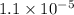
Solution : Given,
Concentration pyridinecarboxylic acid = 0.78 M
pH = 2.53
First we have to calculate the hydrogen ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/2021/formulas/chemistry/high-school/rjo2yhb5oj9ry1fr4db1ujrazm6fh3vhke.png)
![2.53=-\log [H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/jhzrtkvejxdkxfz5i2irvbxovrx89395o9.png)
![[H^+]=2.95* 106{-3}M](https://img.qammunity.org/2021/formulas/chemistry/college/mbstwey8ha9j0crczk4o401m6u2v119r3x.png)
Now we have to calculate the acid dissociation constant.
The equilibrium reaction for dissociation of (weak acid) is,
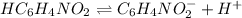
initially conc. 0.78 0 0
At eqm. (0.78-x) x x
The expression of acid dissociation constant for acid is:
![k_a=([C_6H_4NO_2^-][H^+])/([C_6H_4NO_2])](https://img.qammunity.org/2021/formulas/chemistry/college/1fb54bls6iyrxzylbirgkhnzupig2b24zh.png)
As,
![[H^+]=[C_6H_4NO_2^-]=x](https://img.qammunity.org/2021/formulas/chemistry/college/9qdavo67f06vo62f7y4230oxy1cpujz1t7.png)
So,

Now put all the given values in this formula ,we get:
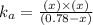
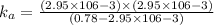
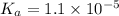
Therefore, the value of acid dissociation constant is,