Answer:
20 ml 13% solution should the professor use
Step-by-step explanation:
Let
the volume of the 13% solution should the professor use = a
So, according to question
13% x a + 18% x (50-a) = 50 x 16%
⇒ 0.13 a + 0.18 x 50 - 0.18 a = 50 x 0.16
⇒ - 0.05 a = 8 - 9
⇒
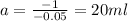
So, 20 milliliters of the 13% solution should be the professor used.
& (50 - 20) = 30 ml of the 18 % solution would be use.