Answer:
The kinetic energy of the electron is
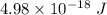 .
.
Step-by-step explanation:
Given that,
The maximum wavelength of a photon is 430 nm.
The frequency of a photon is
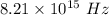
It is a case of photoelectric effect. The relation between the kinetic energy and the the work function as :
So, the kinetic energy of the electron is
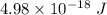 .
.