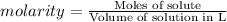Step-by-step explanation:
1) The expression of concentration that provides the moles of solute per kilograms of solvent is molality.
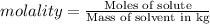
2) If you place 5 moles of sodium chloride and 4 moles of sucrose into 11 moles of water, the mole fraction of sodium chloride would be 0.25.
Mole fraction is the fraction of moles of i-th component to the sum of all moles of components.
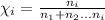
3) A solution is made up of 0.15 grams of sodium chloride in 1 liter of water. For this solution, the solvent is water.
Solution made up two components that is solute and solvent. Amount of solvent is more than amount of solute.
4) A solution is made up of 0.15 grams of sodium chloride in 1 liter of water. For this solution, the solute is sodium chloride.
5) A way to express concentration that provides the moles of solute per liter of solution is molarity .