Answer:
the energy lost by the battery is 233.28Joules
Step-by-step explanation:
Energy = Power × Time
We know that the power is given by :
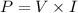
Power = Current (I)×potential difference (V)
Energy lost = current × potential difference × time
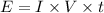
Given that
current, I = 0.018Amperes,
Potential diff ,V = 3.6Volts
timr, t = 1hour = 1×60×60 = 3600seconds
Energy lost. = 0.018×3.6×3600
= 233.28Joules
Hence, the energy lost by the battery is 233.28Joules