Answer:
The rate of change of volume with respect to pressure
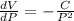
Explanation:
According to boyle,s law When temperature is constant then Pressure is inversely proportional to the volume.
P ∝
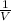
PV = C
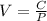
Rate of change of volume with respect to pressure is determined by differentiating the above equation with respect to pressure
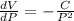
This is the rate of change of volume with respect to pressure.