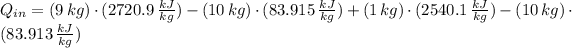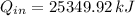Answer:
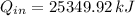
Step-by-step explanation:
Let establish a control volume in the industrial pressure cooker, which is a transient state system. From the First Law of Thermodynamics, the heating process is modelled:
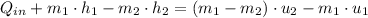
The heat transfered to the cooker is:
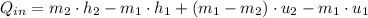
Properties at each state are described below:
State 1
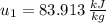
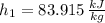
State 2
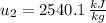
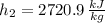
The heat transfered to the cooker is: