Answer:
Excess reactant amount 15.1 moles
Step-by-step explanation:
3 Cu + 8 HNO₃ → 3 Cu(NO₃)₂ + 2 NO +4 H₂O
Using mole ratio method we find limiting reagent and excess reagent
For,
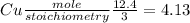
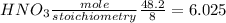
Since, Cu is limiting reagent and HNO₃ excess reagent
According to reaction,
3 moles of Cu react with 8 moles of HNO₃
12.4 moles of Cu react with = 33.06 moles of HNO₃
So remaining amount of excess HNO₃ = 48.2 - 33.06
= 15.1 moles