100g of ammonia releases 271.18 kJ of heat.
Step-by-step explanation:
Given:
N₂ reacts with H₂
The reaction is:
2N₂ + 3H₂ → 2NH₃ + 92.2 kJ
According to the balanced reaction:
2 X 17g of ammonia releases 92.2 kJ of heat
34g of ammonia releases 92.2 kJ of heat
100g of ammonia will release =
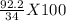 kJ of heat
kJ of heat
= 271.18 kJ
Therefore, 100g of ammonia releases 271.18 kJ of heat.