Answer:
The percentage of silicon atoms per unit volume that are displaced in the single crystal lattice = 0.001 %
The percentage of silicon atoms per unit volume that are displaced in the single crystal lattice with boron atoms = 0.4 ×
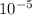 %
%
Step-by-step explanation:
No. of phosphorus atoms = 5 ×
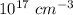
The volume occupied by a single Si atom
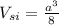
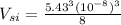
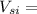 2 ×
2 ×
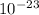
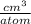
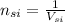
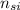 = 5 ×
= 5 ×
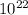
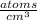
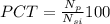
Put the values in above equation we get
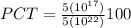
PCT =
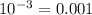 %
%
These are the percentage of silicon atoms per unit volume that are displaced in the single crystal lattice.
(b).
No. of boron atoms = 2 ×
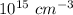
The volume occupied by a single Si atom
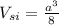
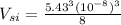
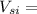 2 ×
2 ×
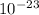
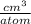
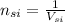
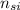 = 5 ×
= 5 ×
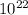
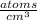
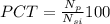
Put the values in above equation we get
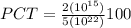
PCT = 0.4 ×
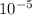 %
%
These are the percentage of silicon atoms per unit volume that are displaced in the single crystal lattice.