Answer:
a) The molecular formula of oleic acid is:
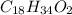
b) There are 5.74 x 10^21 molecules of oleic acid in a 3.00 mL sample.
Step-by-step explanation:
a)
First, let's determine the empirical formula of oleic acid from the percentage composition provided by the question:
- Assuming a 100g sample of oleic acid, the correspondent mass of C, H and O would be:
mass of C = 76.54g
mass of H = 12.13g
mass of O = 11.33g
- We must determine the number of moles contained in each of the masses determined above. For that, let's use the atomic masses of C (12.01 u), H (1.007 u) and O (15.99 u) and the following equation:
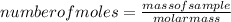
number of moles of C = 76.54g / 12.01 g/mol = 6.373 mol C
number of moles of H = 12.13g / 1.007 g/mol = 12.04 mol H
number of moles of O = 11.33g / 15.99 g/mol = 0.7086 mol O
- Next, we'll try to find whole-number ratios by dividing each number of moles obtained by the smallest value obtained (0.7086):
C = 6.373 / 0.7086 = 8.994 ≅ 9
H = 12.04 / 0.7086 = 16.99 ≅ 17
O = 0.7086 / 0.7086 = 1.000
- Now, we can write the empirical formula of oleic acid as:
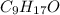
Now that we have the empirical formula for oleic acid (C9H17O), let's check its molar mass and compare it to the experimental value provided by the question (282 g/mol):
molar mass (C9H17O) = 9 * 12.01 + 17 * 1.007 + 1 * 15.99 = 141.9 g/mol
Note that the molar mass obtained from the empirical formula (141.9 g/mol) and the experimental molar mass (282 g/mol) are different, thus we need to find an integer multiple to determine the molecular formula (keep in mind that molecular formulas always present kind and number of atoms of each element present in the molecular compound, while the empirical formula presents the smallest coefficients for each atom).
Molecular formula = n × Empirical formula
This integer multiple, n, can be obtained by dividing the molar mass, MM , of the compound by the empirical formula mass, EFM (the molar mass represented by the empirical formula):
n = MM / EFM
n = 282 / 141.9
n = 1.987 ≅ 2
Since the integer multiple is 2, we can multiply all coefficients in the empirical formula by 2 to find the molecular formula of oleic acid:
C9 H17 O x 2 = C18 H34 O2
Therefore, the molecular formula of oleic acid is:
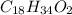
b)
We need to calculate the amount of molecules of oleic acid in a 3.00mL sample, considering the density as 0.895 g/mL.
From the density provided, we can calculate the mass, in grams, of oleic acid contained in 3.00 mL of this compound:
1 mL ----------------------- 0.895 g oleic acid
3.00 mL ----------------- x
Solving for x, we have that there are 2.69 g of oleic acid in a 3.00 mL sample.
Next, considering the molar mass of oleic acid (282 g/mol), we can calculate the number of moles contained in 2.685g of the acid:
282 g -------------------- 1 mol oleic acid
2.69 g ----------------- y
Solving for y, we have that there are 0.00953 moles of oleic acid in a 3.00 mL sample.
Now, using the Avogadro's number (6.022 x 10^23 particles/mol), we can calculate the amount of molecules of oleic acid in the sample:
1 mol oleic acid ------------------------------- 6.022 x 10^23 molecules oleic acid
0.00953 mol oleic acid ------------------- z
Solving for z, we have that there are 5.74 x 10^21 molecules of oleic acid in a 3.00 mL sample.