Answer: The initial volume of the system is 60.29 L.
Step-by-step explanation:
According to the first law of thermodynamics,
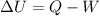
As it is given that heat is being added to the system so,
 will be positive. And, work done on the system is negative and work done by the system is positive.
will be positive. And, work done on the system is negative and work done by the system is positive.
So here,
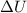 = -107.4 J
= -107.4 J
Q = 52.7 J
P = 0.693 atm
And, W = PdV
or, W =
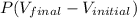
So,
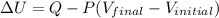
-107.4 J = 52.7 J - 0.693 \times 101.325 (63.2 - V)
-160.1 = -43.79 - 70.21 (63.2 - V)
63.2 - V =
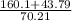
- V = 2.90 - 63.2
V = 60.29 L
Thus, we can conclude that the initial volume of the system is 60.29 L.