Answer:
- 2 moles of glucose would be needed to lower the freezing point of one kg of water 3.72ºC.
Step-by-step explanation:
Lowering the freezing point of a solvent, when a nonvolatils solute is added, is a colligative property that follows the equation:
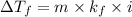
Where:
- ΔTf is the depression on the freezing point
- Kf is the molal kryoscopic constant of the solvent. For water it is 1.86 ºC/m
- i is the van't Hoff factor. For molecular compounds, such as glucose, it is 1.
Then, you can calculate the molality of the solution:
- 3.72ºC = m × 1.86ºC/m × 1
Clear m:
- m = 2 mol of solute /kg of solvent
Since molality is the number of moles per kg of solvent, you would need 2 moles of glucose to lower the freezing point of one kg of water 3.72ºC.