126 grams of H2O is formed.
Step-by-step explanation:
Data given:
volume of the gas = 88 Liters
pressure = 720 mm Hg or 0.947 atm
temperature T = 22 Degrees or 295.15 K
R = 0.08021 atm L/mole K
n =?
The formula is used is of ideal gas law to know the number of moles of CH4 undergoing combustion.
PV = nRT
n =
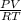
putting the values in the equation
= 0.947 X 88/ 0.08021 X 295.15
n = 3.5 moles
balanced reaction for combustion of methane
CH4 + O2 ⇒ CO2 + 2H20
1 mole of CH4 undergoes combustion to form 2 moles of water
3.5 moles will give x moles of water
2/1 = x/3.5
x = 7 moles of water (atomic mass of water = 18 gram/mole)
mass = atomic mass x number of moles
mass = 18 x 7
=126 grams of water is formed.