Answer:
so correct option is (C) 0.0248 kg/s
Step-by-step explanation:
given data
liquid water = 40°C
steam pressure = 5 MPa
steam temperature = 300°C
steam mass flow rate = 0.5 kg/s
solution
we will apply here energy balance equation that is
m1 × h1 + m2 × h2 = (m1+m2) × h3 ................1
so we can say
m1 × ( h3-h1) - m2× ( h2 - h3) ..............2
and here m1 will be
m1 =
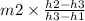 ....................3
....................3
here we know at 40 degree h1 is 167.50 kJ/kg
and for pressure 5MPa we have know saturated temperature is 264 degree
but we have 300 degree that is supersaturated region
so by steam table we get
h3 enthalpy of dry saturated T3 is 2.63.977 degree C
and h3 = h at pressure 5MPa
T = 263.977 so it will 2793.7 kJ/kg
so now put all value in equation 3 we get
m1 =
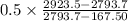
m1 = 0.02471 kg/s
so correct option is (C) 0.0248 kg/s