Answer:
- The researcher's can safely raise the fish before reaching the 170 m depth.
Step-by-step explanation:
Assuming constant temperature, you can use Boyle's law:
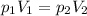
Where:
- p₁ is the pressure at 500 m depth = 50.57 atm
- p₂ is the unknown pressure
- V₂ is the maximum volume that the fish's swim bladder can hold = 0.34 liters
Then, determine p₂:
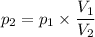
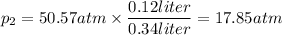
From the table, there is a linear relationship between pressure (atm) and water depth (m).
The slope of the linear function is about 0.099:
- (50 - 40)/(100-50) = 0.099
The y-intercept is 1.00 (the point 0, 1.00).
Then, the linear function is: Pressure = 1 + 0.099×depth
Now you can find the depth at wich the pressure is 17.85 atm
- 17.85atm = 1.00atm + 0.099×depth
- depth = (17.85 atm - 1.00atm) / 0.099 = 170.2 m ≈ 170 m
Hence, the fish's swimm bladder will ruputure at 170m.