Answer:
The value is the temperature of the air inside the tire
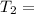 340.54 K
340.54 K
% of the original mass of air in the tire should be released 99.706 %
Step-by-step explanation:
Initial gauge pressure = 2.7 atm
Absolute pressure at inlet
 = 2.7 + 1 = 3.7 atm
= 2.7 + 1 = 3.7 atm
Absolute pressure at outlet
 = 3.2 + 1 = 4.2 atm
= 3.2 + 1 = 4.2 atm
Temperature at inlet
 = 300 K
= 300 K
(a) Volume of the system is constant so pressure is directly proportional to the temperature.
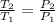
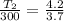
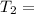 340.54 K
340.54 K
This is the value is the temperature of the air inside the tire
(b). Since volume of the tyre is constant & pressure reaches the original value.
From ideal gas equation P V = m R T
Since P , V & R is constant. So
m T = constant
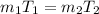
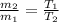
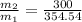
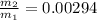
value of the original mass of air in the tire should be released is
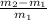
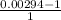
⇒ -0.99706
% of the original mass of air in the tire should be released 99.706 %.