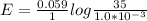Answer:
The voltage is

Step-by-step explanation:
Since the main source of
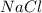 is the sea water then
is the sea water then
The equation for the reaction
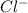 in sea water would be
in sea water would be

that is the ion would form a molecule
 and then release an electron
and then release an electron
For river water
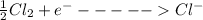
I.e the molecule would gain an electron and forms an ion
Now
 acts as reactant for seawater reaction and product for river water
acts as reactant for seawater reaction and product for river water
The voltage of the concentration cell at initial stage is
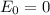
Now at the voltage for the cell with
 concentration difference between sea water and river water is mathematical denoted as
concentration difference between sea water and river water is mathematical denoted as
![E = E_0 - (0.059)/(n_e) log([Cl^-]_(product))/([Cl^-]_(reactant))](https://img.qammunity.org/2021/formulas/chemistry/college/npccltsu5ljkkf8m0pjqi17katc0ef5hgs.png)
Where
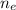 is the number of electron = 1
is the number of electron = 1
So substituting values we have
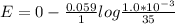
Removing the negative sign