Answer:
A. 10.0 grams of ethyl butyrate would be synthesized.
B. 57.5% was the percent yield.
C. 7.80 grams of ethyl butyrate would be produced from 7.60 g of butanoic acid.
Step-by-step explanation:
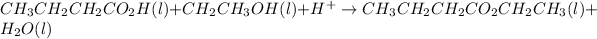
A
Moles of butanoic acid =
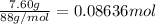
According to reaction ,1 mole of butanoic acid gives 1 mol of ethyl butyrate,then 0.08636 mol of butanoic acid will give :
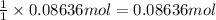 of ethyl butyrate
of ethyl butyrate
Mass of 0.08636 moles of ethyl butyrate =
0.08636 mol × 116 g/mol = 10.0 g
Theoretical yield = 10.0 g
Experimental yield = ?
Percentage yield of the reaction = 100%
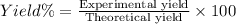
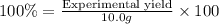
Experimental yield = 10.0 g
10.0 grams of ethyl butyrate would be synthesized.
B
Theoretical yield of ethyl butyrate = 10.0 g
Experimental yield ethyl butyrate = 5.75 g
Percentage yield of the reaction = ?
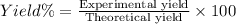
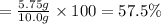
57.5% was the percent yield.
C
Moles of butanoic acid =
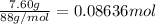
According to reaction ,1 mole of butanoic acid gives 1 mol of ethyl butyrate,then 0.08636 mol of butanoic acid will give :
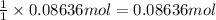 of ethyl butyrate
of ethyl butyrate
Mass of 0.08636 moles of ethyl butyrate =
0.08636 mol × 116 g/mol = 10.0 g
Theoretical yield = 10.0 g
Experimental yield = ?
Percentage yield of the reaction = 78.0%
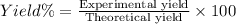
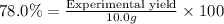
Experimental yield = 7.80 g
7.80 grams of ethyl butyrate would be produced from 7.60 g of butanoic acid.