Answer:
The particles in substance W have a lower average kinetic energy than the particles in substance X.
Step-by-step explanation:
The temperatures of the substances in this problem are:
W X Y Z
12 18 9 20
(expressed in degrees Celsius)
The temperature of a substance is a measure of the average kinetic energy of the particles in the substance.
In fact, the two variables are related by the formula:
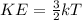
where
KE is the average kinetic energy of the particles
k is the Boltzmann constant
T is the absolute temperature (in Kelvin) of the substance
Therefore, we see that the average kinetic energy of the particles is directly proportional to the temperature of the substance. So, the correct option is
The particles in substance W have a lower average kinetic energy than the particles in substance X.
Because substance W has a lower temperature (12 degrees) than substance X (18 degrees)