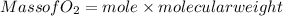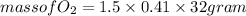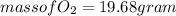Answer:
a:
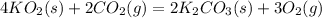
b: Oxygen is the only element which is getting oxidised as well reduced
c:
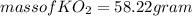 and
and
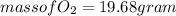
Step-by-step explanation:
Part a: Balanced equation for the given reaction;
Rule:
step1 : first balance the metal
step2: Non metal except oxygen
step 3: lastly balance the oxygen
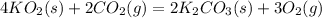
Part b: indicating oxidation number of each element both side
reactant side oxidation number:
K = +1
O= -0.5 and -2
C =+4
Product side oxidation number:
K = +1
O= 0 and -2
C =+4
From the above data it clearly that Oxygen is the only element which is getting oxidised as well reduced .
Part c: Mass calculation
from the balance equation it is clearly that 4 moles of
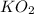 need 2 moles of
need 2 moles of
 for complete reaction i.e. mole
for complete reaction i.e. mole
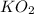 is used double the mole of
is used double the mole of

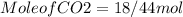
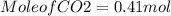
hence the mole of
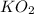 is two times of
is two times of

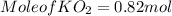
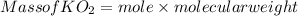
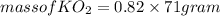
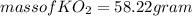
From 2 mole of
 3 mole of
3 mole of
 is produced
is produced
From 1 mole of
 1.5 mole of
1.5 mole of
 is produced
is produced
From 0.41 mole of

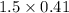 mole of
mole of
 is produced
is produced