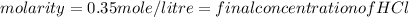Answer:
A: NaOH + HCl = NaCl +H2O
B: Volume of NaOH = 22.2ml
C:
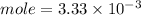
D: Volume of HCl = 9.45 ml
E:
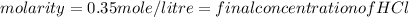
Step-by-step explanation:
Part A: Balanced chemical equaion
NaOH + HCl = NaCl +H2O
part B:
Volume of NaOH used in titration is 22.2 ml because volume is taken upto the end point of the titration.
Part C:
Calculation of moles of NaOH used:
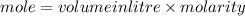
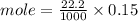
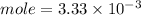
Part D:
Volume of HCl used in titration is 9.45 ml because volume is taken upto the end point of the titration.
Part E:
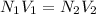
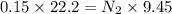

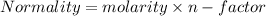
But in case of acid it is known as basicity and in case of base it is known as acidity.
in case of NaOH and HCl n-factor is 1;
hence
Normality=molarity;