Answer:
a person with 75 kg mass will need 4762 ballons
Step-by-step explanation:
The expression for the required number of balloons is
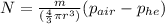
substitute 1.293kg/m^3 for density of air
0.179kg/m^3 for density of helium
75kg for mass m
radius = diameter / 2
= 0.3m / 2
= 0.15m
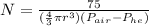
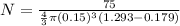
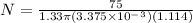
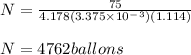
Hence, a person with 75 kg mass will need 4762 ballons