Answer:
The final temperature of the two objects is the same.
Step-by-step explanation:
The expression for the heat energy in terms of mass, specific heat and the change in the temperature is as follows:
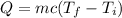
Here, Q is the heat energy, m is the mass of the object, c is the specific heat and
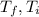 are the final temperature and initial temperature.
are the final temperature and initial temperature.
According to the given question, Two objects of the same mass, but made of different materials, are initially at the same temperature. Equal amounts of heat are added to each object.
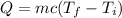 ............(1)
............(1)
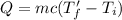 .............(2)
.............(2)
From (1) and (2),
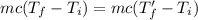
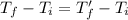
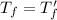
Therefore, the final temperature of the two objects is the same.