Answer:
22.5L
Step-by-step explanation:
Given parameters:
Volume of N₂O₃ = 15liters
Unknown:
volume of oxygen gas = ?
Solution:
To solve this problem, we need to work from the known to unknown. The known is N₂O₃. We can find the number of moles from here.
Note: The condition is Standard temperature and pressure
Number of moles =
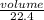
Number of moles of N₂O₃ =
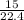 = 0.67moles
= 0.67moles
The equation of the reaction;
2N₂ + 3O₂ → 2N₂O₃
Since the equation is balanced;
2 moles of N₂O₃ is produced from 3 moles of O₂
0.67moles of N₂O₃ is produced from
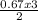 = 1.01 moles of O₂
= 1.01 moles of O₂
Therefore;
Volume of O₂ = 1.01 x 22.4 = 22.5L