84.34 grams of grams of iron (III) chloride that can be produced is maximum because Fe is the limiting reagent in this reaction and chlorine gas is excess reagent.
Step-by-step explanation:
Balanced chemical equation:
2 Fe + 3 Cl2 → 2 FeCl3
DATA GIVEN:
iron = atoms
mass of chlorine gas = 67.2 liters
mass of FeCl3 = ?
number of moles of iron will be calculated as
number of moles =
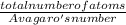
number of moles =
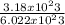
number of moles = 0.52 moles of iron
moles of chlorine gas
number of moles =
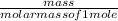
Putting the values in the equation:
n =
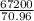 (atomic mass of chlorine gas = 70.96 grams/mole)
(atomic mass of chlorine gas = 70.96 grams/mole)
= 947.01 moles
Fe is the limiting reagent so
2 moles of Fe gives 2 moles of FeCl3
0.52 moles of Fe will give
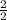 =
=
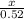
0.52 moles of FeCl3 is formed.
to convert it into grams:
mass = n X atomic mass
= 0.52 x 162.2 (atomic mass of FeCl3 is 162.2grams/mole)
= 84.34 grams