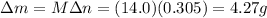Answer:
4.27 g
Step-by-step explanation:
We can write that for a gas kept at constant pressure and temperature, the number of moles of the gas is directly proportional to its volume:
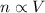
Which can be rewritten as
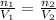
where here we have:
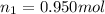 is the initial number of moles
is the initial number of moles
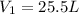 is the initial volume of the gas
is the initial volume of the gas
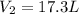 is the final volume of the gas
is the final volume of the gas
So the final number of moles is
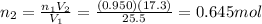
So the number of moles that should be released from the balloon is:
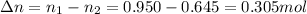
The molar mass of gas N2 is
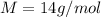
Therefore, the mass of gas that should be released is: