Answer:
12.4L
Step-by-step explanation:
Given parameters:
T₁ = 225K
T₂ = 350K
V₁ = 8L
Unknown:
V₂ = ?
Solution:
Since this heating takes place under constant pressure, we can resolve this temperature and volume relationship using Charles's law.
According to this law "the volume of a gas is directly proportional to the temperature provided that pressure is constant".
Mathematically, we have;
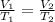
Input the parameters and solve for the unknown;
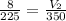
V₂ = 12.4L