Answer:
205.3°C
Step-by-step explanation:
Given parameters:
V₁ = 0.287L
V₂ = 0.18L
T₂ = 37°C
Unknown:
T₁ = ?
Solution:
Since we are interested in volume and temperature relationships in a fixed pressure of the balloon, Charles's law will be a perfect solution to this problem.
Charles's law states that "At constant pressure, the volume of a fixed mass of gas is directly proportional to its temperature".
Mathematically;
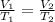
where V and T are volume and temperature of the gas
1 and 2 are initial and final states;
let us convert T₂ = 37°C to K; 37 + 273 = 300K
Input the parameters and solve for the unknown;
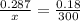
T₁ = 478.3K
Now convert back to °C; 478.3 - 273 = 205.3°C