Answer:
The missing species is carbon-12
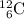 .
.
The nuclear equation should be
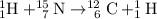 .
.
Step-by-step explanation:
Let
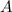 represent the mass number of the missing species, and let
represent the mass number of the missing species, and let
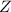 represent its atomic number.
represent its atomic number.
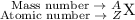 .
.
For this question, there are three things to consider:
- The sum of the mass numbers should be conserved.
- Since there's no beta particle (
 or
or
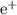 ) involved, the sum of the atomic numbers should also be conserved.
) involved, the sum of the atomic numbers should also be conserved. - The atomic number of the missing species should correspond to atomic symbol.
Mass Number
The sum of the mass numbers on the left-hand side of this reaction is:
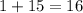 .
.
The sum of the mass number on the right-hand side (including that of the missing species) is
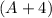 .
.
These two numbers should be the same. In other words,
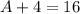 . Therefore, the mass number of the missing species would be
. Therefore, the mass number of the missing species would be
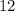 .
.
Atomic Number
The sum of the atomic numbers on the left-hand side of this reaction is:
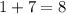 .
.
The sum of the mass number on the right-hand side (including that of the missing species) is
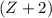 .
.
These two numbers should be the same. In other words,
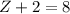 . Therefore, the mass number of the missing species would be
. Therefore, the mass number of the missing species would be
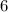 .
.
Symbol
The atomic number
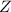 of the missing species is
of the missing species is
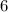 . Look up a modern periodic table. The element with atomic number
. Look up a modern periodic table. The element with atomic number