1.64 L of SO₂ is produced when 2.35 g of Sulfur burns.
Step-by-step explanation:
First of all, we need to write the balanced equation as,
S (s) + O₂ (g) → SO₂ (g)
Now we have to find the number of moles of sulfur by using its given mass and molar mass.
1 mol of Sulfur atoms = 32 g of sulfur
Now the given mass is 2.35 g.
So
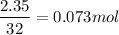
1 mol of S produces 1 mol of SO₂.
So 0.073 mol of S produces 0.073 mol of SO₂.
At STP, 1 mol of SO₂ occupies a volume of 22.4 L.
0.073 mol of SO₂ occupies a volume of 22.4 × 0.073 = 1.64 L
So 1.64 L of SO₂ is produced.