Answer:
Step-by-step explanation:
Use the ideal gas equation:
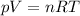
Where:
- p is pressure: 0.950atm
- V is volume: unknown
- n is number of moles: unknown
- R is the universal constat of gases: 0.08206 atm.liter/ (K.mol)
- T is the absolute temperature: 345K
Use the molar mass of the gas to include the density in the formula:
- molar mass = mass in grams / number of moles
- ⇒ mass in grams = number of moles × molar mass
- density = mass in grams / volume
- ⇒ density = number of moles × molar mass / volume
- density = (n/V) × molar mass
- ⇒ n/V = density / molar mass
Clear n/V from the gas ideal equation and subsittute with density/molar mass:
- density / molar mass = n/V
- density/molar mass = p/(RT)
- molar mass = density × RT / p
Now you can subsitute the data:
molar mass = (3.50g/liter) × 0.08206 atm.liter/(K.mol) × 345K / 0.950 atm
- Round to the nearest whole number: 104g/mol ← answer