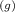Answer:
CH₄
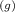 + 2O₂
+ 2O₂
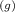 → CO₂
→ CO₂
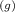 + 2H₂O
+ 2H₂O
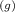
Step-by-step explanation:
This kind of reaction in which a hydrocarbon combines with oxygen gas is a combustion reaction. They are mostly used to produce heat energy that can be transformed into other forms of energy.
Every chemical reaction is made up of reactants and products. The combining species on the left hand side are the reactant and the products are the result of the combination.
Note: in writing chemical equations, the state of matter must always be included.
Reactants : Methane gas CH₄
Oxygen gas O₂
Product: Carbon dioxide CO₂
Water vapor H₂O
CH₄
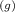 + 2O₂
+ 2O₂
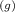 → CO₂
→ CO₂
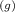 + 2H₂O
+ 2H₂O