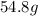Answer:
Step-by-step explanation:
1. Calculate the number of moles of NiCl₂
a) Identify the known variables
- V =0.800 liters
- M = 0.531M
- n = ?
b) Formula
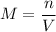
c) Clear n
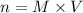
g) Substitute and compute
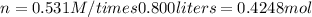
2. Convert moles to grams
a) molar mass of NiCl₂
- 129g/mol (shown in the problem)
b) Unit canceling method
Use the factors in order to cancel the units to obtain grams
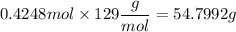
Note that the mol unit appears on the numerator and denominator, so it cancels leaving just g (grams).
c) Round to 3 signficant figures