The final temperature after absorbing the energy of 990 J is 25.014 °C.
Step-by-step explanation:
The specific heat capacity formula can be used to solve the given problem.
So here the heat absorbed or the Q value is given as 990 J. Then the mass of the sample of water m is given as 59 g. The initial temperature is said to be 21 °C and the final temperature has to be determined.
The specific heat capacity is given as 4.18 J/g°C.
Then,
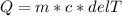
So, the difference in temperature or the change in temperature has to be determined first.
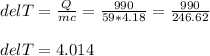
So, the change in temperature = ΔT = T₂-T₁ = 4.014 °C
T₂-21 = 4.014
T₂ = 4.014+21 = 25.014 °C.
So, the final temperature after absorbing the energy of 990 J is 25.014 °C.