Answer:
volume of
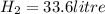
Step-by-step explanation:
Firstly balance the given chemical equation,
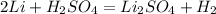
From the given balance equation it is clearly that,
2 mole of Li gives 1 mole of H2 gas
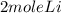 ⇔
⇔
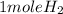
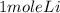 ⇔
⇔
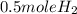
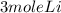 ⇔
⇔
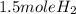
hence
3 mole of Li will give 1.5 mole H2 gas
therefore volume of gas produced from 3 mole Li at
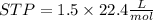
volume of H2=33.6 litre